A Case Report of Gallbladder Adenocarcinoma with Presentation Mimicking Klatskin Tumor Underwent Liver Transplantation
Author(s): PIROUZ SAMIDOUST , MAZIAR MOAYERIFAR, ATHAR ZAMANI, MANI MOAYERIFAR, MAHBOOBEH GHOLIPOUR, FAREHEH KHOSRAVI LARIJANI, ARASH DARYAKAR, FATEMEH FARHADI, SABA MOUSAVI-GUILANI, MEHRZAD RAHMANIAN
*Corresponding author: Dr. Mehrzad Rahmanian, Department of Cardiovascular surgery, Imam Khomeini Hospital Complex, Tehran University of Medical Sciences, Tehran, Iran; E-mail: Mehr2rah@yahoo.com
Citation: Samidoust P, Moayerifar M, Zamani A, Patel B, Moayerifar M, Larijani FK, et al. (2024) A Case of Gallbladder Adenocarcinoma with Presentation Mimicking Klatskin Tumor Underwent Liver Transplanta- tion: A Case Report. Clin Img and Med Case Rep Vol.1 No.2
Copyright: ©2024 Samidoust P, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
We report the case of a 36-year-old male patient with progressive jaundice. Although imaging reported a Klatskin tumor, the patient was later diagnosed with gallbladder adenocarcinoma. Physicians must consider gallbladder adenocarcinoma as a differential diagnosis of Klatskin tumor and perihilar mass even radiologic findings don’t show any abnormality of the gallbladder
Keywords
Adenocarcinoma, Gallbladder, Klatskin tumor, Carcinoma, Case report
Introduction
The sixth most common tumor of the gastrointestinal system and the first most common malignant tumor of the biliary system is Gallbladder cancer (GBC). GBC more occurs in the seventh decade, with a markedly regional variation in incidence and approximately two to six times more frequent in women. The frequent histological type of this cancer is adenocarcinoma (1). Most cases present with right upper quadrant or epigastric pain with jaundice, nausea and vomiting, anorexia, and weight loss (2). In addition to the aggressive nature of tumor and its anatomical location, nonspecific presentation may result in delayed diagnosis at an advanced stage (3). In cases of suspected GBC, ultrasonography (USG) is the initial imaging modality. However, in early disease, USG may not detect any abnormality (2). Hence, the most commonly used evaluative imaging technique is the CT scan, which can reveal polypoid masses, focal or diffuse wall thickening, or, a mass replacing the gallbladder (4).
Herein, we describe a gallbladder adenocarcinoma which was misdiagnosed as hilar cholangiocarcinoma, named Klatskin tumor in a 36-year-old man with no abnormality finding in gallbladder at radiologic assessment and negative Fine Needle Aspiration (FNA) for malignancy at preoperative work up.
Case report
A 36-year-old man presented at the tertiary care facility with a chief complaint of progressive jaundice and generalized itching from two years ago. Based on the patient’s medical history, jaundice originated from the sclera and subsequently progressed to a generalized state over time. He also suffered from anorexia and experienced significant weight loss. The patient’s past medical history did not reveal any prior illnesses and he didn’t take any medications. In social history, he mentioned smoking and using opium. On physical examination, vital signs were stable. His Body mass index (BMI) was standard. Icteric sclera was noticed and there was no evidence of hepatomegaly, splenomegaly, or mass on abdominal examination.
The laboratory tests for Viral Hepatitis showed normal results, while elevated levels of Alkaline Phosphatase, Total Bilirubin, Direct Bilirubin, Aspartate Aminotransferase (AST), and Alanine Transaminase (ALT) were observed (Table 1). The first ultrasonography (US) of the liver and gall bladder revealed an ill-defined hyperechoic lesion measuring about 46*29 mm in segment VI of the liver at the periportal region; the Intrahepatic duct was irregular and dilated in this region, which was suspected to be hilar cholangiocarcinoma (Klatskin tumor); The Gallbladder was containing small amount of sludge and the wall thickness was normal. For further evaluations, Endoscopic ultrasound (EUS) was performed, which showed a wall thickness at the proximal part of the common hepatic duct and the proximal part of the common bile duct along with a mass forming thickness at the hilum of liver that was suggestive for hilar cholangiocarcinoma; The Pancreas and left lobe of the liver were normal, three lymph nodes were observed at the hilum of the liver, the largest was measured up to 15*20mm in diameters. EUS guided FNA from hepatic hilar mass was performed with 22-gauge needle. The cytology report one week later revealed that the received sample was free of malignant cells. Based on FNA result, first he received three-month course of corticosteroid therapy for suspected autoimmune hepatitis, which may lead to lymphadenopathy of the liver hilum and its subsequent symptoms, but due to the lack of therapeutic effects and increased Bilirubin level, a Percutaneous Trans Hepatic cholangiography (PTC) tube was inserted and a spiral computed tomography (CT) scan was conducted to further evaluate the abdominal and pelvic regions. The scan revealed that the liver is of normal size. In segments VI and VII of the liver, a 58*32 mm hypodense lesion with an ill-defined margin compatible with known carcinoma was observed. Mild dilation of the intrahepatic bile duct and distended gallbladder was also seen. A second FNA-guided EUS procedure was conducted, and once again the result showed no presence of malignant cells. Magnetic resonance imaging (MRI) of the liver both with and without contrast, identified a lesion in the hilum of the liver spanning 30*22 mm. The lesion exhibited delayed enhancement, which strongly indicates the presence of cholangiocarcinoma (specifically, a Klatskin tumor) (Figure 1). Due to the strong suspicion of malignant hilar cholangiocarcinoma (Klatskin tumor Type IV) Multidisciplinary Team of Transplant Committee, candidated the patient for a liver transplant after receiving neoadjuvant therapy for six sessions. After neoadjuvant chemotherapy, radiologic findings did not report increasing size of the tumor or metastasis.
After neoadjuvant therapy Bilirubin level decreased and the disease didn’t not progress. Because The waiting time for transplant was long, two more sessions were used for neoadjuvant therapy. patient underwent a liver transplant finally. During the surgery, they noticed the adhesion of the Klatskin tumor to the duodenum, and for unblock resection multidisciplinary team performed radical cholecystectomy, hepatectomy and Pancreaticoduodenectomy (Wipple surgery). The sample sent to the pathology (Figure 2). In the recovery unit, the patient awoke uneventfully and was transferred to the intensive care unit. On the fourth day after surgery, he began a clear liquid diet and tolerated oral feeding, and after ten days, he was transferred to the ward. The patient underwent adjuvant therapy, and follow-up was scheduled for him one month later.
Table 1 Viral laboratory data
| HCV Ab | Negative | Total Bilirubin | 24.2 |
| HBS Ag | Negative | Direct Bilirubin | 17.3 |
| HIV Ab | Negative | AST | 373 |
| EBV Ab | Negative | ALT | 635 |
| CA 19-9 | 40.3 | ALP | 4350 |
| CEA | 4.2 | LDH | 955 |
Hepatitis C virus Antibody, Hepatitis B antibody, Human Immunodeficiency virus antibody, Epstein-Barr virus antibody, Carbohydrate antigen 19-9, Cancer Embryonic antigen, Aspartate aminotransferase, Alanine transaminase, Alkaline phosphatase, Lactate dehydrogenase
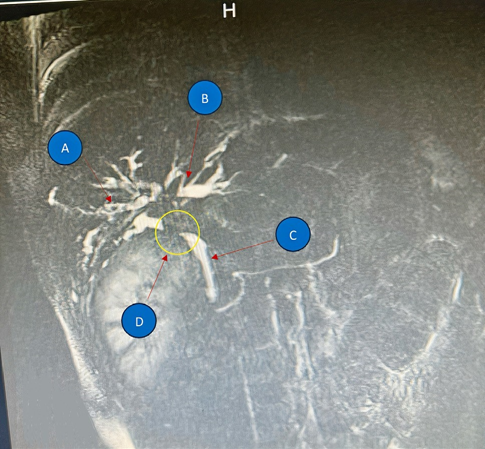
Figure 1: The Sagital plane of Liver MRI with contrast. (A) right hepatic duct; (B) left hepatic duct; (C) Common bile duct; (D) Filling defect in common hepatic duct (Klatskin tumor type IV).
Klatskin tumor to the duodenum, and for unblock resection multidisciplinary team performed radical cholecystectomy, hepatectomy and Pancreaticoduodenectomy (Wipple surgery). The sample sent to the pathology (Figure 2). In the recovery unit, the patient awoke uneventfully and was transferred to the intensive care unit. On the fourth day after surgery, he began a clear liquid diet and tolerated oral feeding, and after ten days, he was transferred to the ward. The patient underwent adjuvant therapy, and follow-up was scheduled for him one month later.
The Microscopic exam of tissue sample showed neoplastic tissue composed of irregular glands lined by cells with atypical vesicular cigar-shaped nuclei, conspicuous nucleoli, and acidophilus cytoplasm bearing frequent mitosis infiltrating through inflamed desmoplastic stroma extended to adjacent pancreatic tissue (Figure 3). In order to determine tumor origin, Immunohistochemistry (IHC) study performed (Table 2). And finally, Histomorphology and IHC results are suggestive of moderately differentiated gallbladder adenocarcinoma. Tumor size was 7*3 cm (in gallbladder) and involved liver hilum (m:6*2.5 I’m, pT3) and the staging was T3N0MX (at least IIIA).
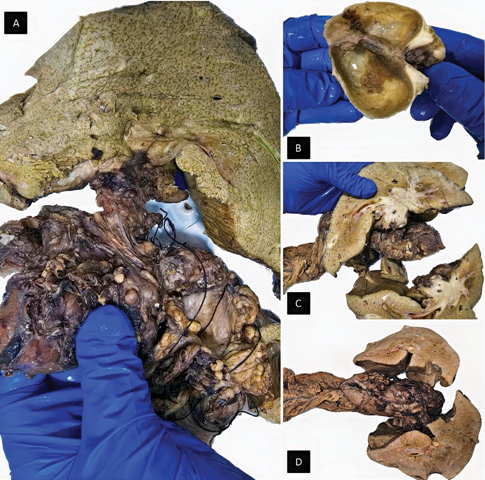
Figure 2. (A) Total hepatectomy; (B) cholecystectomy with evidence of focal gallbladder wall thickening; cut surfaces of liver show heterogenous mass with necrosis; (C) cut surfaces of liver show heterogenous mass with necrosis (D) pancreaticoduodenectomy (Whipple) specimens.
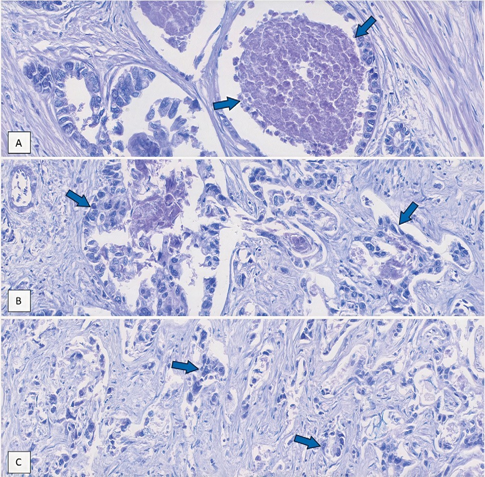
Figure 3: (A): Irregular/ angulated glands lined by polygonal atypical tumoral cells with evidence of necrosis, (B): Tumoral cells have enlarged nuclei showing vesicular chromatin, prominent nucleoli, and acidophilic cytoplasm, (C): The adenocarcinoma is moderately differentiated; morphologically it consists of poorly formed glands and is surrounded by desmoplastic stroma.
Discussion
Gallbladder cancer continues to be a leading cause of death in some nations (5). It affects 64% of Caucasians, 17% of Hispanics, 9% of African Americans, and 2% of Pacific Islanders/Asians (6,7). About 70% of cases are found in women. Advanced age, female sex, obesity, cholelithiasis or other benign gallbladder pathology, porcelain gallbladder, persistent infection with Helicobacter pylori or Salmonella species, abnormal pancreaticobiliary duct junction and gallbladder polyps are the factors most frequently linked to gallbladder cancer (6-9). Gallstones are one of the risk factors for developing GBC, and 85% of patients have them (10). In our case, the patient was male, and the age of the patient was 36 years old, not obese, and had no history of gallbladder pathology or infection with Helicobacter pylori.
Table 2: Immunohistochemistry Studies
| CK7 | Negative | Synaptophysin | Negative |
| CK19 | Positive | Chromogranin | Negative |
| CK20 | Negative | TTF1 | Negative |
| Hepatocyte | Negative | Napsin A | Negative |
| Arginase I | 40.3 | PAX 8 | Negative |
| CDX2 | 4.2 | SATB2 | Positive |
Early detection of gallbladder cancer is crucial since it may improve the prognosis. Anorexia, weight loss, an abdominal lump, and jaundice are common indicators of advanced stages (11). Compared to the pathologic progression, the clinical presentation of gallbladder cancer is typically unclear or delayed, which results in advanced staging and a poor prognosis at the time of diagnosis (12–14). Because the symptoms of GBC are ambiguous and might mimic other illnesses, early identification has historically been challenging and typically delayed. In some patients TH elevated levels of tumor markers such as Cancer Embryonic antigen (CEA) and Carbohydrate antigen 19-9 (CA 19-9) may be noticed, although they are not specific to the gallbladder (15).
Serum tumor indicators, such as CA 19-9 and CEA, are considered as valuable diagnostic aids for certain malignancies and as instruments to evaluate the efficacy of therapeutic interventions. The role of CA 19-9 in management of hepatobiliary and pancreatic malignancies holds considerable importance, while its reliability remains a topic of debate. CEA functions as a notable biomarker in several forms of carcinoma, including colorectal, gastric, gallbladder, breast, urinary tract, and lung carcinoma. It is commonly employed in the diagnostic evaluation of patients who are suspected of malignancies. Serum concentrations of CA 19-9 above 1,000 U/ml and CEA serum levels that are fourfold higher than the standard serum value of 3.6 ng/ml are highly suggestive of malignancy. In our case, the serum concentration of CA19-9 was low for malignancy but the serum level of CEA was 4.2 ng/ml and was suspected for malignancy (16). Samidoust P, et al.
Increased alkaline phosphatase in serum may occur after gallbladder cancer progression to bile duct obstruction, but rarely before it spreads or advanced (15). This was indicated in our case, in which the 36-year-old male patient had been experiencing jaundice for two years, and laboratory data findings demonstrated elevated AST, ALT, ALP, and CA 19-9.
Aggressive gallbladder cancer typically invades the adjacent organs and disseminates to distant places, At the time of initial discovery, 40-65% of patients with GBC may have a mass occupying lesion (8,14). Imaging methods including contrast enhanced CT and MRI can identify GBC as focal or diffuse asymmetric wall thickening. The CT scan of gallbladder carcinoma (GBC) exhibits distinct characteristics, including the presence of a distinct focal mass within the gallbladder, uneven thickening of the gallbladder wall, and a “2-layer pattern” of enhancement observed in the thickened wall of the gallbladder. Additionally, it may exhibit other characteristics such as the infiltration of adjacent organs, the presence of localized lymphadenopathy, and the formation of metastatic deposits in the liver and peritoneum (14-17).
Several pathological conditions, including tuberculosis, sarcoidosis, lymphoma, and metastasis, exhibit the presence of enlarged lymph nodes surrounding the hepatic hilum. These findings may lead to misdiagnosis of advanced Klatskin tumors. In addition, certain conditions such as primary sclerosing cholangitis, intrahepatic stones, and oriental cholangiohepatitis may resemble a Klatskin tumor or an early-stage cholangiocarcinoma. Therefore, it is important to thoroughly assess patients who are suspected of hilar cholangiocarcinoma in order to detect individuals with specific conditions that do not need surgical intervention (18).
The study conducted by Tsalis et al. (16) presented a case of Klatskin tumor in a female patient, which was initially suspected but later confirmed to be metastatic hilar lymphadenopathy caused by rectal cancer. Additionally, they identified a male patient with a Klatskin tumor who exhibited enlarged lymph nodes in the porta hepatis, ultimately leading to a diagnosis of right-colon cancer (16). Diagnosing metastatic lymphadenopathy in the hepatic hilum requires careful evaluation, including a detailed medical history and current symptoms.
In our case all imaging findings demonstrated perihilar mass, that seemed to have developed within a lymph node, and misled the diagnosis towards a Klatskin tumor Type IV. Bismuth-Corlette classification categorizes Klatskin tumors based on anatomical sites in the biliary tree. Type IV is defined as multicentric tumors or involvement of the confluence and both right and left hepatic ducts (19).
The radiologic finding also reported that gallbladder contained sludge. In this case, the results of the EUS-guided FNA of hepatic hilar mass also reported Negative for malignant cells. Because there was a high suspicion of a Klatskin tumor, the multidisciplinary team decided to perform Whipple surgery and liver transplant. Before the surgery, 8 sessions of chemotherapy were planned and after chemotherapy, radiology findings did not report increasing size of the tumor or metastasis. In our case, multidisciplinary team performed radical cholecystectomy, hepatectomy and Pancreaticoduodenectomy (Wipple surgery). After surgery, the sample was sent to the pathology, and the pathology report showed that Tumor was T3N0MX and IHC reported it was a gallbladder adenocarcinoma (Figure 2).
Conclusion
In the case described in this report, a young man who was previously healthy presented with two years of progressive jaundice and significant weight loss. Based on radiologic and laboratory workup, it was believed that the patient had perihilar cholangiocarcinoma, also known as a Klatskin tumor, but after a Whipple procedure and liver transplant, the patient discovered with gallbladder adenocarcinoma. Before the diagnosis, the patient underwent a Whipple surgery and a thorough workup. Preoperative diagnosis of Klatskin tumors requires thorough evaluation of patient’s medical history, physical examination, hematological test results, and radiographic imaging. Physicians must maintain suspicion for alternative conditions similar to Klatskin tumors. Being under follow-up of an oncologist and a mental health specialist is crucial in the care of a rare occurrence like the case presented. Collaboration between surgeons, radiologists, pathologists, and any specialist whenever the presentation is suspected may maximize the diagnosis of the disease prior to surgery.
Consent
Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal on request.
Declaration of Interest
Nothing to declare.
Funding source
This research did not receive any specific grant form funding agencies in the public, commercial, or not-for- profit sectors.
Ethical Approval
Not required for case reports at our hospital. Single case reports are exempt from ethical approval in our institutions.
References
- Halaseh SA, Halaseh S, Shakman R (2022) A review of the etiology and epidemiology of gallbladder cancer: What you need to know. Cureus. 14(8).
- Kanthan R, Senger JL, Ahmed S, Kanthan SC (2015) Gallbladder cancer in the 21st century. Journal of oncology. 2015.
- Zou Z, Yan J, Zhuge Y, Chen J, Qian X, Liu B (2016) Multidisciplinary collaboration in gallbladder carcinoma treatment: A case report and literature review. Oncology Letters 12: 2696–701.
- Vijayakumar A, Patil V, Mallikarjuna MN, Shivaswamy BS (2012) Early diagnosis of gallbladder carcinoma: an algorithm approach. ISRN radiology 2013.
- Aloia TA, Járufe N, Javle M, Maithel SK, Roa JC, Adsay V, et al. (2015) Gallbladder cancer: expert consensus statement. Hpb 17: 681–690.
- Hickman L, Contreras C (2019) Gallbladder cancer: diagnosis, surgical management, and adjuvant therapies. Surgical Clinics 99: 337-355.
- Sharma A, Sharma KL, Gupta A, Yadav A, Kumar A (2017) Gallbladder cancer epidemiology, pathogenesis and molecular genetics: Recent update. World journal of gastroenterology 23: 3978.
- Jia Y, Samadzadeh S, Cornford M, Ji P, French SW (2020) Educational case: incidental gallbladder adenocarcinoma. Academic Pathology. 7: 2374289520909504.
- Hundal R, Shaffer EA (2014) Gallbladder cancer: epidemiology and outcome. Clinical epidemiology. 99–109.
- Rawla P, Sunkara T, Thandra KC, Barsouk A (2019) Epidemiology of gallbladder cancer. Clinical and experimental hepatology 5: 93–102.
- Sampetoding S, Kusuma MI, Pratiwi Y, Ulfandi D, Faruk M (2022) Gallbladder adenocarcinoma with upper abdominal pain: A case report. International Journal of Surgery Case Reports 100: 107734.
- Adachi T, Haraguchi M, Irie J, Yoshimoto T, Uehara R, Ito S, et al. (2016) Gallbladder small cell carcinoma: a case report and literature review. Surgical Case Reports 2: 1–5.
- Bains L, Maranna H, Lal P, Kori R, Kaur D, Mallya V, et al. (2021) The giant resectable carcinoma of gall bladder—a case report. BMC surgery 21: 1–7.
- Andrén-Sandberg Å. (2012) Diagnosis and management of gallbladder cancer. North American journal of medical sciences 4: 293.
- Jefferys D, Roy S, Majid A (2022) Incidental adenocarcinoma of the gallbladder in a patient with Y insertion gallbladder duplication in the context of recurrent biliary colic: A video case report. Medicine 101.
- Tsalis K, Parpoudi S, Kyziridis D, Ioannidis O, Savvala NA, Antoniou N, et al. (2019) Klatskin tumors and “Klatskin- mimicking lesions”: our 22-year experience. Rev Esp Enferm Dig 111: 121–128.
- Mitchell CH, Johnson PT, Fishman EK, Hruban RH, Raman SP (2014) Features suggestive of gallbladder malignancy: analysis of T1, T2, and T3 tumors on cross-sectional imaging. Journal of computer assisted tomography 38: 235.
- Senthil Kumar MP, Marudanayagam R (2012) Klatskin-like lesions. HPB Surgery 2012.
- Brunicardi FC (2019) Schwartz’s principles of surgery. McGraw-Hill Education.

