Axial Spondyloarthritis and IgA Vasculitis: Rare Association or Just Not Recognized
Author(s): ROBIN SIA, MUEED MIAN
Department of Rheumatology, The Northern Hospital, Epping, Victoria, Australia
*Corresponding author: Robin Sia, Department of Rheumatology, The Northern Hospital, Epping, Victoria, Australia; Tel: +619342 8561; E-mail: robinsiawaijen@gmail.com
Citation: Sia R (2024) Axial Spondyloarthritis and IgA Vasculitis: Rare As- sociation or Just Not Recognized. Clin Img and Med Case Rep Vol.1 No.2
Copyright: ©2024 Sia R, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Categories: Rheumatology, Internal Medicine, General Medicine
Keywords: Axial spondyloarthritis, Ankylosing spondylitis, IgA vasculitis
Axial spondyloarthritis (axSpA) also known as ankylosing spondylitis is an inflammatory arthritis involving the spine and sacroiliac joints with extra-musculoskeletal manifestations most commonly involving the eye, gut and skin. Other organ involvement can occur including cardiac (aortic insufficiency), lungs (upper-lobe predominant interstitial fibrosis) and kidneys (nephritic syndrome). Immunoglobulin A vasculitis (IgAV) formally known as Henoch-Schönlein purpura [HSP] is the most common form of systemic vasculitis in children and is often self-limited. The disease is characterized by palpable purpura, arthritis or arthralgia, abdominal pain as well as kidney involvement. It has been proposed that there is a selective increase of serum IgA levels during active inflammatory disease of axSpA. Here we explore a case of a gentleman who presents with IgA vasculitis and was found to have a new diagnosis of axSpA. We have also reviewed the current literature of cases of IgAV on the background of patients with ankylosing spondylitis.
Introduction
Axial spondyloarthritis (axSpA) also known as ankylosing spondylitis (AS) is a chronic inflammatory disease involving the axial skeleton and can also cause peripheral manifestations such as asymmetrical oligoarthritis, enthesitis as well as dactylitis (1). Although the association between seronegative spondyloarthropathies and IgA Nephropathy (IgAN) has been previously documented, the association with IgA Vasculitis (IgAV) remains unclear with only ten reported cases found in our literature review. Furthermore, among the cases found, axSpA with negative HLA-B27 and IgAV is extremely rare with only three reported cases. This article discusses a case of newly diagnosed IgAV with an incidental diagnosis of HLA-B27-negative radiological axSpA (r-axSpA) in a gentleman as well as to review the current literature and highlights the relationship between these two conditions.
Case Presentation
We report a case of a gentleman in his 50s who presented with abdominal and testicular pain, haematuria as well as purpuric rash. He first developed a rash on his ankles bilaterally 4 days prior, then subsequently involving his distal upper limbs. The rash was palpable and was not painful, nor pruritic (Figure 1A). There were no recent upper respiratory tract or infective symptoms nor any recent medication changes. In regards to the scrotal tenderness and swelling, there was no dysuria, discharge or recent trauma.
His background history includes an idiopathic right bronchial rupture in 2022 where he underwent embolization. He does not take any regular medications. He lives with his mother and is independent in all his activities of daily living, and does not smoke or drink alcohol.
On examination, his vital signs were within normal limits. On further examination, there was non-blanching palpable purpuric rash noted at his distal legs and proximal arm. His testicular exam demonstrates a swollen and tender right testicle.
Investigations
If relevant
Blood tests demonstrated a normal full blood count as well as liver function test and kidney function. His C-Reactive protein (CRP), urine albumin:creatinine ratio (uACR) and urine protein/creatinine ratio (uPCR) were elevated with no glomerular red cells (Table 1). His urine culture and sensitivity showed staphylococcus aureus however given he was systemically well with no infective prodrome or signs of bacteraemia, this was thought to be a contaminant by the infectious disease team and was not treated.
Further imaging was done including a scrotal ultrasound which confirmed a right sided epididymitis with a moderate hydrocele, a CT-IVP which showed moderate left hydroureteronephrosis secondary to a 10 mm obstructing calculus at the left pelviureteric junction, and associated urothelial thickening, enhancement and adjacent stranding, with incidental fusion of the sacroiliac joints (SIJs) bilaterally (Figure 1B). An x-ray of his sacroiliac joints was subsequently performed which demonstrated bilateral sacroiliitis estimated at grade 4 on the right and grade 3 on the left (Figure 1C).
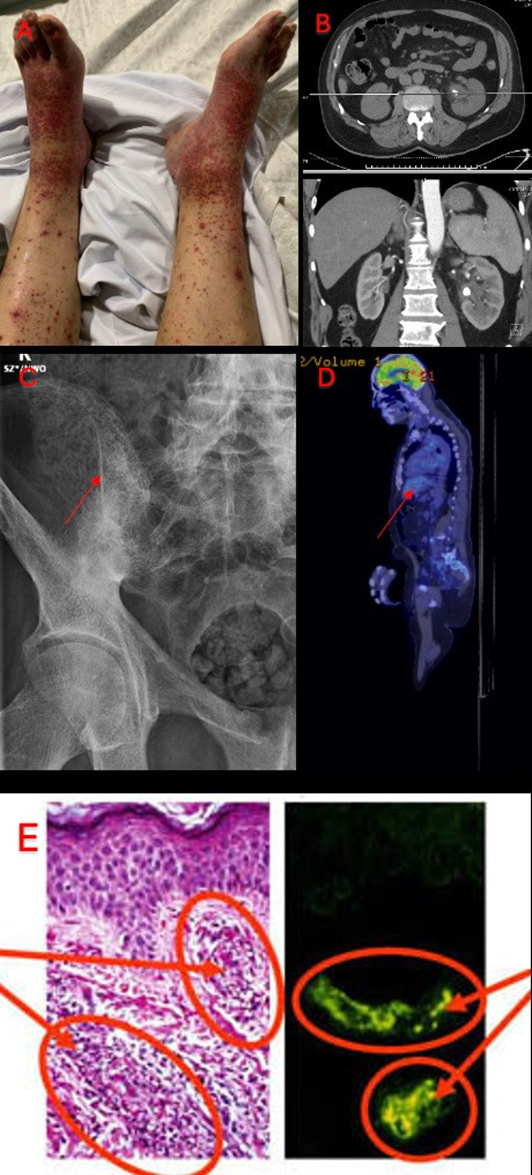
Figure 1A: Non-blanching petechiae on the patient’s bilateral distal lower limbs
Figure 1B: CT-IVP which showed moderate left hydroureteronephrosis secondary to a 10 mm obstructing calculus at the left pelviureteric junction, and associated urothelial thickening, enhancement and adjacent stranding
Figure 1C: X-ray of SIJ demonstrating bilateral sacroiliitis estimated at grade 4 on the right and grade 2-3 on the left
Figure 1D: FDG-PET scan showing an incidental mass highly suspicious for a GIST with lack of FDG avidity
Figure 1E: Histopathology of skin biopsy with the left image demonstrating leukocytoclastic vasculitis with a positive immunofluorescence stain of IgA on the right
CT-IVP: CT-intravenous pyelography; FDG-PET: Fludeoxyglucose-18 positron emission tomography; GIST: gastrointestinal stromal tumour; IgA: Immunoglobulin A
Following his incidental finding of bilateral sacroiliitis, a targeted history revealed that he has had ongoing inflammatory sounding lower back pain over the past 10 years with minimal peripheral joint involvement. The pain was worse in the mornings, with morning stiffness lasting more than 30 minutes. There was no known history of psoriasis, inflammatory bowel disease or anterior uveitis.
He also denies any new sexual partners. He also occasionally takes non-steroidal anti-inflammatories (NSAIDs) with improvement in his back pain. His examination further demonstrates an occiput-to-wall distance of 1cm, modified Schober’s test of 3cm, with restriction on frontal and lateral spinal flexion without evidence of SIJ tenderness. There were no extra-articular manifestations.
An autoimmune screen was subsequently sent which demonstrated a positive anti-nuclear antibody (ANA), elevated erythrocyte sedimentation rate (ESR) and IgA levels (Table 1). Other relevant negative tests include a negative extractable nuclear antigen antibody (ENA), rheumatoid factor (RF), anti-citrullinated protein antibody (ACPA), antistreptolysin O titer (ASOT), normal C3/ C4 levels and dsDNA levels as well as negative anti- glomerular basement membrane (GBM) antibodies. Further tests showed a negative pANCA and cANCA, with a negative antiphospholipid screen and normal angiotensin converting enzyme (ACE) levels. HLA-B27 testing was done which was negative. A positron emission tomography (PET) scan was done in the setting of his constellation of disease and positive findings which demonstrated an incidental mass with lack of FDG avidity highly suspicious for a gastrointestinal stromal tumour (GIST) (Figure 1D). A skin biopsy of his rash was done which demonstrated leukocytoclastic vasculitis with a positive immunohistochemistry stain of IgA (Figure 1E).
Table 1: Patient’s lab investigations. eGFR, estimated glomerular filtration rate.
| Investigation | Value | Normal range |
|---|---|---|
| Haemoglobin (g/L) | 124 | 115-155 |
| White cell count (x10^9/L) | 8.2 | 4.0-12.0 |
| Platelet count (x10^9/L) | 174 | 150-400 |
| eGFR | >90 | >90 |
| Creatinine (umol/L) | 78 | 45-90 |
| Urea (mmol/L) | 7.5 | 3.5-8.0 |
| Sodium (mmol/L) | 136 | 135-145 |
| Potassium (mmol/L) | 4.1 | 3.5-.5.2 |
| ALP (U/L) | 67 | 30-110 |
| GGT (U/L) | 35 | <37 |
| ALT (U/L) | 33 | 5-35 |
| AST (U/L) | 30 | <31 |
| CRP (mg/L) | 94 | <6 |
| uACR (mg/mmol) | 26.6 | <2.5 |
| uPCR (mg/mmol) | 37 | <30 |
| ESR (mm/hr) | 62 | <21 |
| CK (u/L) | 51 | 30-150 |
| ANA | 1:160 (Speckled) | <1:80 |
| IgA (g/L) | 10.6 | 0.6-4.6 |
| IgG (g/L) | 16.9 | 7.0-16.5 |
| IgM (g/L) | 0.7 | 0.4-2.7 |
ALP: Alkaline Phosphatase; GGT: Gamma glutamyl transferase; ALT: Alanine aminotransferase; AST: Aspartate aminotransferase; CRP: C-Reactive Protein; uACR: Urine albumin:creatinine ratio; uPCR: Urine protein:creatinine ratio; ESR: Erythrocyte sedimentation rate; CK: Creatine kinase; ANA: anti-nuclear antibody; IgA: Immunoglobulin A; IgG: Immunoglobulin G; IgM: Immunoglobulin M.
Differential Diagnosis
If Relevant
A few differentials were considered initially including ANCA-associated vasculitis but given no other stigmata of conditions such as granulomatous polyangiitis (GPA), microscopic polyangiitis (MPA) and eosinophilic granulomatous polyangiitis (eGPA) with negative ANCA titres, this was ruled out. Infectious causes could trigger this but blood cultures remained negative and the patient was afebrile. Haematological aetiologies such as thrombotic microangiopathy (TMA) such as Immune Thrombocytopenic Purpura (ITP) was considered, but the platelets remained normal. Drug-induced leukocytoclastic vasculitis could contribute to this presentation but given no recent exposure to sulphur drugs and the presence of IgA on immunohistology staining makes this more likely to be IgA Vasculitis.
Treatment
Treatment If Relevant
He was commenced on 20mg prednisone with a weaning dose by 5mg weekly to continue 5mg ongoing until Rheumatology outpatient appointment. In regards to his incidental gastric lesion, it was stable compared to the imaging in December 2022 without PET avidity and was booked for an outpatient gastroscopy as well as for a rigid cystoscopy with left pyeloscopy with lithotripsy given the renal calculi.
Discussion
Include a very brief review of similar published cases
Axial spondyloarthritis (axSpA) is a chronic inflammatory disease with a predilection for involving the axial skeleton and encompasses both radiographic (r-axSpA) and non-radiographic axSpA (nr-axSpA). The average age of onset is usually in the third decade of life with a male to female ratio of 2:1 for r-axSpA and equal sex distribution for nr-axSpA (1). There is an estimated prevalence of 0.1% to 1.4%, with a higher incidence in populations with high background prevalence of HLA-B27 such as Northern Europe, Eurasia and North America and low prevalence in southern Africa and Japan (2). The primary site of involvement of axSpA is enthesis and in the subchondral bone (3,4). Genetics play a major role in pathogenesis of this disease, with a heritability estimated to be 90% with HLA-B27 as the main risk factor as well as other major histocompatibility complex (MHC) and non-MHC variants such as endoplasmic reticulum aminopeptidase (ERAP) and interleukin-23 receptor (5,6). Multiple factors contribute to the progression of axSpA, including mechanical stress, damage to the skin in patients with psoriasis and to the gut microbiome with dysbiosis in intestinal mucosa in inflammatory bowel disease (4-9). These leads to axial inflammation, bone destruction as well as new bone formation driven by tumour necrosis factor (TNF)-α and IL-23/IL-17 (2).
There has been some documented increase in IgA levels in patients as well as in the skin with axSpA. The mean serum IgA was 38% higher in patients with axSpA compared to control (10,11). Although the association between IgAV and Familial Mediterranean Fever (FMF) is well-documented, the association with SpA remains unknown (1). The following table includes all the reported cases of IgAV and concomitant axSpA, with majority of cases being HLA-B27 positive (Table 2).
The first two cases documented by Peeters et al. discusses a new diagnosis of IgA vasculitis in patients with inflammatory bowel disease (IBD) and axSpA, with immunofluorescence studies showing perivascular deposition of immunoglobulin A in the skin biopsies of both patients as well as in the renal mesangium of one of the patients. Furthermore, IgA immune complexes were found in the serum samples of the patients (12). Skin changes are not characteristic of axSpA compared to IBD which include erythema nodosum, erythema multiforme, pyoderma gangrenosum, psoriasis, nodular necrobiosis and epidermolysis bullosa, and rarely cutaneous polyarteritis nodosa and granulomatous vasculitis (12). These cases highlight the possibility of IgA as a possible pathogenesis. Furthermore, it has been proposed that the concept of abnormal IgA secretion via microbial antigenic stimulation of the digestive mucosa plays a role in the pathogenesis of ankylosing spondylitis (13). IgAV can often be diagnosed with skin biopsies demonstrating leukocytoclastic vasculitis (LCV) with IgA deposits. LCV is defined as small vein vasculitis and can be associated with autoimmune conditions, drugs (penicillin, sulfonamides, NSAIDs, thiazides, retinoids and quinolones) and infections. Kobak et al. supports the fact that ankylosing spondylitis can be one of the causes of LCV and that treatment of this condition relies on treating the cause (14,15).
Table 2: Characteristics of cases reported in the literature of patients with concomitant IgAV and axSpA [1].
| Authors | Year | Sex | Age (years) | HLA-B27 status | Active axSpA |
|---|---|---|---|---|---|
| Peeters et al. [12] | 1990 | Male | 35 | Negative | No |
| Peeters et al. [12] | 1990 | Male | 50 | Negative | No |
| Beauvais et al. [13] | 1990 | Male | 50 | Negative | Yes |
| Beauvais et al. [13] | 1993 | Male | 45 | Positive | Yes |
| Machet et al. [14] | 1997 | Male | 31 | Positive | Yes |
| Kobak et al. [15] | 2014 | Male | 26 | Positive | Yes |
| John et al. [16] | 2019 | Male | 26 | Positive | Yes |
| Kamath et al. [17] | 2022 | Male | 40 | Positive | No |
| Demouveaux et al. [18] | 2024 | Male | 25 | Positive | No |
| Demouveaux et al. [18] | 2024 | Male | 30 | Positive | No |
axSpA: axial spondyloarthritis; NA: non-available; NSAID: non-steroidal anti-inflammatory drugs; RCT: randomised clinical trial
The idea of elevated IgA levels among patients with axSpA has led to further studies to explore the use of IgA antibodies in early axSpA (16-19). Furthermore, the role of serum IgA levels are important in several aspects, such as the fact that regular treatment with NSAIDs can reduce serum IgA levels, suggesting that NSAIDs may have a disease modifying effect in axSpA. Serum IgA levels could eventually play a role in monitoring disease activity alongside erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP) in clinical practice (20).
Finally, there has only been 10 reported cases of axSpA associated with IgAV, with eight in prior literature review and additional two reported in the study by Demouveaux et al. (18).
Learning Points/Take Home Messages
3-5 bullet points
- This case report and literature view emphasizes the rare association between ankylosing spondylitis and IgA vasculitis (IgAV)
- Although the relationship between these two conditions is still unclear, evidence have demonstrated that given the elevated levels of IgA in patients with axSpA, there may be a higher risk to develop IgAV.
- The differentials for cutaneous vasculitis are broad, but skin biopsies remain an important aspect as one of our diagnostic tools.
- Clinicians should have a low threshold to recognize and diagnose IgAV in patients with a background history of axSpA. Early recognition of IgAV is essential as renal involvement may occur.
- There may be a role of the use of therapeutics that may target both conditions, especially given the limitations in treatment options for IgAV.
References
- Navarro-Compán V, Sepriano A, El-Zorkany B, van der Heijde D (2021) Axial spondyloarthritis. Ann Rheum Dis 80: 1511-1521.
- Stolwijk C, van Onna M, Boonen A, van Tubergen A (2016) Global Prevalence of Spondyloarthritis: A Systematic Review and Meta-Regression Analysis. Arthritis Care Res (Hoboken) 68: 1320-1331.
- Schett G, Lories RJ, D’Agostino MA, Elewaut D, Kirkham B, Soriano ER, et al. (2017) Enthesitis: From pathophysiology to treatment. Nat Rev Rheumatol 13: 731-741.
- Watad A, Bridgewood C, Russell T, Marzo-Ortega H, Cuthbert R, McGonagle D (2018) The early phases of ankylosing spondylitis: Emerging insights from clinical and basic science. Front Immunol 9: 2668.
- Ellinghaus D, Jostins L, Spain SL, Cortes A, Bethune J, Han B, et al. (2016) Analysis of five chronic inflammatory diseases identifies 27 new associations and highlights disease-specific patterns at shared loci. Nat Genet 48: 510-518.
- Cortes A, Hadler J, Pointon JP, Robinson PC, Karaderi T, Leo P, et al. (2013) Identification of multiple risk variants for ankylosing spondylitis through high-density genotyping of immune-related loci. Nat Genet 45: 730-738.
- Ansell RC, Shuto T, Busquets-Perez N, Hensor EMA, Marzo- Ortega H, McGonagle D (2015) The role of biomechanical factors in ankylosing spondylitis: The patient’s perspective. Reumatismo 67: 91-96.
- Ramiro S, Landewé R, Van Tubergen A, Boonen A, Stolwijk C, Dougados M, et al. (2015) Lifestyle factors may modify the effect of disease activity on radiographic progression in patients with ankylosing spondylitis: A longitudinal analysis. RMD Open 1: e000153.
- Van Praet L, Van Den Bosch FE, Jacques P, Carron P, Jans L, Colman R, et al. (2013) Microscopic gut inflammation in axial spondyloarthritis: A multiparametric predictive model. Ann Rheum Dis 72: 414-417.
- Cowling P, Ebringer R, Ebringer A (1980) Association of inflammation with raised serum IgA in ankylosing spondylitis. Ann Rheum Dis 39: 545-549.
- Collado A, Sanmarti R, Bielsa I, Castel T, Kanterewicz E, Ca?ete JD, et al. (1988) Immunoglobulin A in the skin of patients with ankylosing spondylitis. Ann Rheum Dis 47: 1004-1007.
- Peeters AJ, Van Den Wall Bake AWL, Daha MR, Breedveld FC (1990) Inflammatory bowel disease and ankylosing spondylitis associated with cutaneous vasculitis, glomerulonephritis, and circulating IgA immune complexes. Ann Rheum Dis 49: 638-640.
- Beauvais C, Kaplan G, Mougenot B, Michel C, Marinho E (1993) Cutaneous vasculitis and IgA glomerulonephritis in ankylosing spondylitis. Ann Rheum Dis 52: 61-62.
- Machet L, Jan V, Ouakil H, Vaillant L, Esteve E, Lorette G (1997) Cutaneous leukocytoclastic vasculitis in a case of ankylosing spondylitis. Acta Dermato-Venereologica 77: 324. Sia R, et al.
- Kobak S, Yilmaz H, Karaarslan A, Yalcin M (2014) Leukocytoclastic Vasculitis in a Patient with Ankylosing Spondylitis. Case Rep Rheumatol 2014: 653837.
- John KJ, Sadiq M, Thomas M, Turaka VP (2019) Henoch- Schonlein purpura associated with HLA-B27 positive axial spondyloarthritis in a young man. BMJ Case Rep 12: e228881.
- Kamath S, Ahmed T, Rana F, Upadhyay AS (2022) Rare case of ankylosing spondylitis complicated by IgA vasculitis. BMJ Case Rep 15: e252182.
- Demouveaux A, Delclaux M, Goudot A, Duponchelle E, Cortet B, Flipo RM, et al. (2024) Association of axial spondyloarthritis and IgA vasculitis: Report of two cases. JointBoneSpine 91: 105723.
- Ruytinx P, Vandormael P, Quaden D, Luyten E, Geusens P, Vanhoof J, et al. (2023) Antibodies of the immunoglobulin a isotype to novel antigens in early axial spondyloarthritis. Front Med (Lausanne) 9: 1072453.
- Franssen MJAM, Van de Putte LBA, Gribnau FWJ (1985) IgA serum levels and disease activity in ankylosing spondylitis: A prospective study. Ann Rheum Dis 44: 766-771.

